Nylon Fibers Educational Research for the Apparel & Textile Industry
Monika Kannadaguli and Ramaiah Kotra
INTRODUCTION
Nylon was the first truly synthetic fiber to be commercialized (1939). Nylon was developed in the 1930s by scientists at Du Pont, headed by an American chemist Wallace Hume Carothers (1896-1937). It is a polyamide fiber, derived from a diamine and a dicarboxylic acid, .Because a variety of diamines and dicarboxylic acids can be produced, there are a very large number of polyamide materials available to produce nylon Fibers. The two most common versions are nylon 66 (polyhexamethylene adiamide) and nylon 6 (Polycaprolactom, a cyclic nylon intermediate). Raw materials for these are variable and sources used commercially are benzene (from coke production or oil refining), furfural (from oat hulls or corn cobs) or 1,4-butadiene(from oil refining). The chemical reaction are as following [1]:
Chemical reaction
fiber types are produced commercially in various parts of the world. Nylon 66 has been preferred in North American markets, whereas nylon 6 is much more popular in Europe and elsewhere. Nylon is produced by meltspinning and is available in staple, tow, monofilament, and multi-filament form. The fiber has outstanding durability and excellent physical properties. Nylons are semi-crystalline polymers. The amide group -(-CO-NH-)- provides hydrogen bonding between polyamide chains, giving nylon high strength at elevated temperatures, toughness at low temperatures, combined with its other properties, such as stiffness, wear and abrasion resistance, low friction coefficient and good chemical resistance. These properties have made nylons the strongest of all man-made Fibers in common use. Because nylons offer good mechanical and thermal properties, they are also a very important engineering thermoplastic. For example, 35% of total nylon produced is used in the automobile industry [2]. There are several commercial nylon products, such as nylon 6, 11, 12, 6/6, 6/10, 6/12, and so on. Of these, the most widely used nylon products in the textile industry are formed of nylon 6 and nylon 6/6. The others are mainly used in tubing extrusion, injection molding, and coatings of metal objects [3].
Nylon's outstanding characteristic in the textile industry is its versatility. It can be made strong enough to stand up under the punishment tire cords must endure, fine enough for sheer, high fashion hosiery, and light enough for parachute cloth and backpacker's tents. Nylon is used both alone and in blends with other Fibers, where its chief contributions are strength and abrasion resistance. Nylon washes easily, dries quickly, needs little pressing, and holds its shape well since it neither shrinks nor stretches.
fiber Formation
One of the most important factors in polymer processing is viscosity, which is a function of molecular weight. The number-average molecular weight of polymer suitable for textile fiber production ranges from 14,000 to 20,000. Since polycaprolactam can be regarded at equilibrium as a polycondensation polymer, the number-average molecular weight alone is sufficient for its characterization. Two-step melt spinning, comprised of spinning and drawing, is considered to be the conventional method to manufacture nylon filaments. After melting, filtering, and deaerating, the molten polymer is extruded through a spinneret into a chamber where the melt solidifies into a filament form. At this stage, the filaments have little molecular orientation, and their slight birefringence is due to shear forces set up during extrusion. In order to achieve desirable properties through molecular orientation and crystallinity, the newly formed filaments must be drawn. Since the Tg of nylon is below room temperature, nylon can be cold drawn.
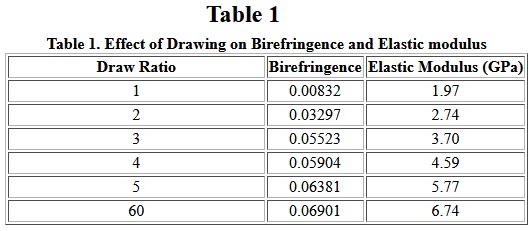
Hot drawing is also frequently used. Nylon filaments are drawn approximately four times their initial length. The effect of drawing on birefringence, a measure of molecular anisotropy, can be seen in Table I. Also, the elastic modulus increases significantly with increasing orientation as shown in Table I. Other physical properties, such as density equilibrium, moisture sorption, tenacity and elongation-at-break, are also affected by drawing.
Table I
Rather than two-step spinning (extrusion) and drawing, a one-step, high-speed spinning process is being used increasingly. In high-speed spinning, filament windup speed relative to the extrusion speed is very high and orientation and crystallization occur in elongation flow along the spin line. When drawing as-spun Fibers, the molecules are arranged randomly in amorphous regions and as folded chains in crystalline region as shown in Figure 1.
Figure 1 - sorry, the Figure 1 is no longer available for view.
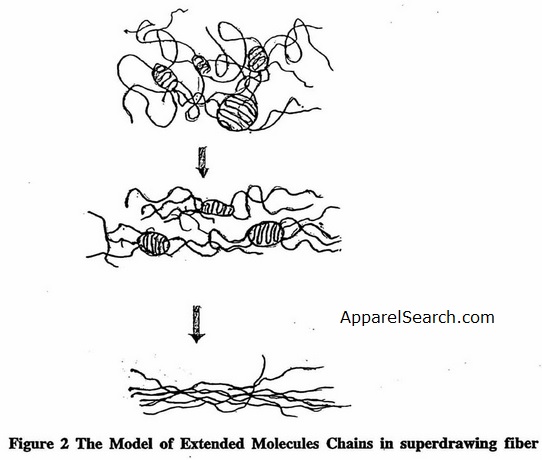
In essence, cold drawing stretches chains in amorphous regions, but molecular folds are restricted and the molecules orient themselves along the fiber axis direction, resulting in enhanced orientation and high crystallinity. In the case of nylons, which have sheet-like crystal structures, drawing may enable the hydrogen-bonded polyamide sheets to slip past each other and form more oriented structure [4]. Hot drawing is a procedure using high temperature during drawing and annealing under restraint after drawing. Exposure to high temperature helps to increase the draw ratio, and higher moduli and tenacity can be achieved.. Ultradrawing of solidified crystalline material induces a high degree of chain extension (Figure 2, which leads to very high tensile strength and modulus. This results in a so-called high-performance fiber.
Figure 2
A skin-core structure, mostly depending on spinning speed, is generally formed within melt-spun Fibers. At a constant feeding rate, higher spinning speeds will produce more extended chains in the melt and form a finer filament. Therefore, the finer fiber usually has higher modulus and tenacity. Fine filament cannot be drawn as much as a coarse filament, because partial orientation on the outer parts of the filaments is formed when the molten fluid is drawn over the sides of the orifice. As a result, finer filaments have a greater proportion of 'skin' to bulk, i.e., better orientation has already been formed. Naturally, there is not much space for an improvement by cold drawing within fine filaments. The filaments become lustrous and strong.
Rheological Behavior
The melt viscosity of the polymer can be represented as a function of molecular weight by the relationship [5, 6]:
=K(Mw)a
where is the zero shear viscosity, Mw it the weight average molecular weight, K and a are constants dependent upon the polymer and temperature. In the case of nylons, the value of exponent a normally is in the range of 3.4-3.8.
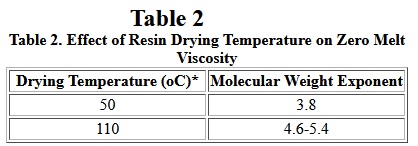
It has long been known that moisture has a strong effect on the rheological behavior of nylons. Generally, high moisture levels cause degradation and foaming, and relatively low levels of moisture act as plasticizer in nylon 6 during melt processing. All nylons absorb moisture. the extent of moisture absorption depends on temperature, crystallinity, and humidity. Therefore, before processing of nylon resins, the polymer pellets must be dried to moisture levels below 0.2 wt%, in order to avoid bubble formation and significant polymer degradation during processing. A recent study [7] found that zero shear melt viscosity is affected by the drying temperatures used. The result is shown in Table II.
Table II
Non-conventional Spinning Techniques
Alternative to conventional melt spinning, various solution spinning techniques have been introduced [8,9]. Solution spinning techniques (gel, wet, dry) enable the spinning of high molecular weight polyamides, leading to high tenacity filaments (tenacity 100cN/tex)[8].
As an innovation on fiber formation, new technologies producing microfiber have been developed and reported [10]. MicroFibers are produced primarily by direct spinning and mechanical and solvent splitting. Electrospinning [11] represents another approach to fiber spinning, when electrical forces on polymer melt or solution surface overcome the surface tension and cause an ejection from an electrically charged jet. The diameter of the Fibers produced by this technique is of the order of nanometers. Frequently, there are produced Fibers that are electrically charged.
Crystalline structure
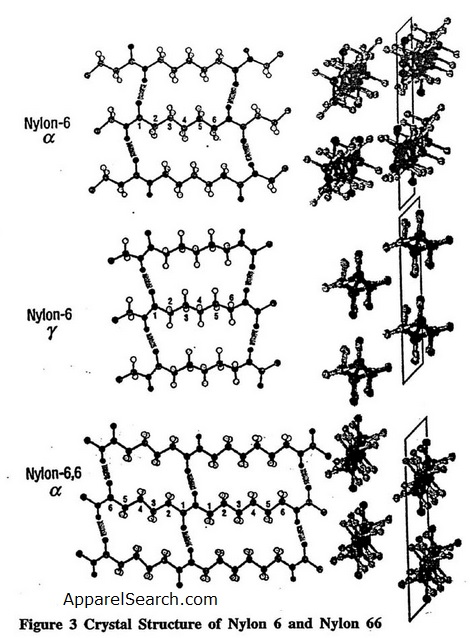
Both nylon 6 and nylon 66 are semi-crystalline polymers. These linear aliphatic polyamides are able to crystallize mostly because of strong intermolecular hydrogen bonds through the amide groups (Figure. 3)[3], and because of van der Waals forces between the methylene chains. Since these unique structural and thermo-mechnical properties of nylons are dominated by the hydrogen bonds in these polyamides, quantum chemistry can be used to determine the hydrogen bond potential[3]. The left side of the figure shows hydrogen-bonding planes, and the right side shows the view down the chain axis. For the -form of nylon 6, adjacent chains are antiparallel and the hydrogen bonding is between adjacent chains within the same sheet (bisecting the CH2 angles). For the -form of nylon 6, the chains are parallel and the hydrogen bonding is between chains in adjacent sheets. . In nylon 66, the chains have no directionality. Research results have shown that the stable crystalline structure is the -form comprised of stacks of planar sheets of hydrogen-bonded extended chains. It also appears that Young's modulus of the -form is higher than the -form.
Figure 3
Mechanical, thermal and optical properties of Fibers are strongly affected by orientation and crystallinity. Basically, higher fiber orientation and crystallinity will produce better properties. Crystallinity of nylons can be controlled by nucleation, i.e., seeding the molten polymer to produce uniform sized smaller spherulites. This results in increased tensile yield strength, flexural modulus, creep resistance, and hardness, but some loss in elongation and impact resistance. Another important benefit obtained from nucleation is decrease of setup time during processing [1].
Dyeability
The dyeability of nylon Fibers is enhanced due to the end groups -COOH and -NH2, which exhibit polar and hydrophilic characteristics. Dye diffusion into Fibers is closely related to the rate of dyeing, level of dyeing through dye migration, wet fastness properties of dyes, etc. It is generally believed that dye diffusivity is independent on dye concentration, with some exception. T. Shibusawa [12] studied the diffusion of most disperse dyes on nylon 6 and found that the actual diffusivity on nylon 6 Fibers is not always independent on dye concentration. Kim et al. [13] have reported that both dyeing rate and dye saturation of 1,4 -diaminoanthraquinone (1,4-DAA) were improved considerably in the presence of didodecyldimethlammonium bromide (DDDMAB). The amount of DDDMAB adsorbed on nylon 6 fiber is roughly 20 times higher than that a conventional dispersing agent. This suggests that there might be fairly strong interaction between DDDMAB and the fiber by virtue of electrostatic and hydrophobic interactions.
There have been many attempts to improve nylon's dyeability or at least to point out the factors and mechanisms acting in nylon dyeing. It has been shown that acrylonitrile and styrene radiation grafting on the polymer could improve the dyeability of nylon[14]. Another approach to higher dyeability of nylon 6 is by copolymerization [15]. In this case, the dyeability can be improved at the expense of a decrease of specific viscosity and of heat and hydrolysis resistance.
Other treatments, such as plasma etching [16] and superheated steaming [17] have proved to decrease nylon dyeability. In the former treatment, outer structures, not normally susceptible to dyes, are etched away whereas the crystalline phases inside the fiber are not as much affected. Superheated steaming of the Fibers leads to higher shrinkage and to higher crystallinity and crystal size, which contribute to decrease dyeability.
Degradation
The -COOH and -NH2 end-groups in nylons are sensitive to light, heat, oxygen, acids and alkali. When exposed to elevated temperatures, unmodified nylons undergo molecular weigh degradation, which results in loss of mechanical properties. The degradation is highly time/temperature dependent. By adding heat stabilizer, nylon can be used at elevated temperature for long-term performance. Exposure to UV light results in degradation nylon over an extended period of time, it appears that adding carbon black can reduce the radiation degradation. Nylons are chemical resistance to hydrocarbons, aromatic and aliphatic solvents, but they are attacked by strong acids, bases, and phenols. They also are gradually attacked hydrolytically by hot water. Newly developed sulfonation of nylon 6 fiber [18] by 2,5 dichlorobenzene sulfonyl chloride (DSBC) has a great effect on the heat and chemical stability of the Fibers. It reported that the modified fiber is non-melting up to 1000oC, and does not burn when put it in direct flame (but chars without losing fiber form). It does not dissolve in formic acid and concentrated mineral acid. Its glass transition temperature is about 500oC.
Properties of Nylon 66
-Tenacity-elongation at break ranges from 8.8g/d-18% to 4.3 g/d-45%. Its tensile strength is higher than that of wool, silk , rayon, or cotton.
- 100% elastic under 8% of extension
-Specific gravity of 1.14
-Melting point of 263oC
-Extremely chemically stable
-No mildew or bacterial effects
-4 - 4.5% of moisture regain
-Degraded by light as natural Fibers
-Permanent set by heat and steam
-Abrasion resistant
-Lustrous- Nylon Fibers have the luster of silk
-Easy to wash
-Can be precolored or dyed in wide range of colors, dyes are applied to the molten mass of nylon or to the yarn or finished fabric.
-Resilient
-Filament yarn provides smooth, soft, long lasting fabrics
-Spun yarn lend fabrics light weight and warmth
Properties of Nylon 6
The main difference between nylon 6 and nylon 6,6 is nylon 6 has a much lower melting point than nylon 66. This is a serious disadvantage, as garments made from it must be ironed with considerable care.
Nonwovens Usage
The fiber has outstanding durability and excellent physical properties. Like PET fiber, it has a high melting point, which conveys good high- temperature performance. The fiber is more water sensitive than PET; despite this fact, nylon is not considered a comfortable fiber in contact with the skin. Its
toughness makes it a major fiber of choice in carpets, including needlepunched floor-covering products.
Because of its relatively high cost, nylon has somewhat limited use in nonwoven products. It is used as a blending fiber in some cases, because it conveys excellent tear strength. The resiliency and wrinkle recovery performance of a nonwoven produced from nylon is not as excellent as that from PET fiber.
In certain applications, the performance of nylon fiber is hard to beat. However, because of its higher cost, it is used in specialized applications where its performance can justify the increased cost. It is used as a blending fiber in some cases, because it conveys excellent tear strength. The resiliency and wrinkle recovery performance of a nonwoven produced from nylon is not as excellent as that from PET fiber. This polymer is used in moderate quantities, because it is more expensive than polyester, polypropylene, or rayon. Some particular applications are as follows:
- It can be mostly found in garment interlinings and wipes where it supplies strength and resilience.
- In Ni/h and Ni/cd batteries, nylon Fibers are used as nonwovens separators.
- Nylon Fibers are used for the manufacture of splittable-pie Fibers. These Fibers find application in high performance wipes, synthetic suede, heat insulators, battery separators and speciality papers.
- Nonwovens developed from nylon are found in automotive products, atheletic wear and conveyor belts. >
References
[1]. P. G. Galanty, and G. A. Bujtas, Modern Plastics Encyclopedia '92, pp 23-30 McGraw Hill 1992
[2]. P. Meplestor, Modern Plastics, 74, Jan., 66 (1997)
[3]. S. Dasgupta, W.B.hammond, and W.A. Goddard III, J. Am. Chem. Soc. 118,12291-12301, (1996)
[4] A. B. Thompson fiber structure Edited by J. W. Hearle and R. H. Peters, Butterworth & Co. Ltd. and the Textile Institute pp 499 (1963)
[5]. E.Mukouyama and A. Takegawa, Kobunshi Kagaku, 13,323 (1956)
[6]. G. Pezzin and G. B. Gechele, J. Appl. Polym. Sci., 8, 2159 (1964)
[7] Y. P. Khanna, P. K. Han, and E. D. Day, Polym. Eng. and Sci., 36,1745-1754 (1996)
[8]. J. Smook, G. J. H. Vos, and H. L. Doppert, J. Appl. Polym. Sci., 41,105-116 1990)
[9]. J. W. Cho, G. W. Lee, and B. C. Chun, J. Appl, Polym. Sci., 62(5),771-8 (1996)
[10]. D. Gintis, Chemical Engineering World, 32(3),43-50 (1997)
[11]. D H. Reneker, and I. Chun, Nanotechnology, 7(3), 216-23 (1996)
[12]. T. Shibusawa Textile Research Journal, 66,421-428 (1996)
[13]. I. Kim, E. Kono, and T. Takagishi, Textile Research Journal, 66, 763-770 (1996)
[14]. K. El Salmawi, M. B. El Hosamy, A. M. El Naggar, and A. M. El Gendy, American Dyestrff Reporter, 82 (5),47-59 (1993)
[15]. M. Nagata and T. Kiyotsukuri, European Polymer Journal, 28 (9),1069-72 (1992)
[16]. T. Okuno, T. Yasuda, and H. Yasuda, Textile Research Journal, 62(8),474-80 (1992)
[17]. L. Han, T. Wakida, and T. Takagishi, Textile Research Journal, 57(9),519-12 (1987)
[18]. S. A. El Garf and S. M. El kemry, Textile Research Journal, 67, 13-17 (1997)
Apparel Search
Add Your Company Contact
Us About Us Advertise
News Letter Legal
Help
Copyright ©
1999-2023 Apparel Search Company. All Rights Reserved.
Buy Fashion
For The Holidays.